-

-
Harold H Zakon
Professor
Department of Neuroscience, Department of Integrative BiologyThe function, regulation, and evolution of voltage-dependent ion channels. The regulation of sodium and potassium channels by hormones.h.zakon@austin.utexas.edu
Phone: 512-471-0194
Office Location
PAT
Postal Address
2415 SPEEDWAY
AUSTIN, TX 78712-
Harold Zakon received his Ph.D. in Neurobiology & Behavior from Cornell University in 1981. He did postdoctoral research at the Scripps Institution of Oceanography, which is part of the University of California at San Diego from 1981-1983. He joined the faculty of the former Zoology Department at the University of Texas in 1983. He has been Chairman of the Section of Neurobiology since it was founded in 1999. He has served on grant review panels at the NIH, editorial boards of a number of scientific journals and advisory boards of scientific societies. He has won awards for his research, such as an NIH Research Career Development Award and a Research Award from the Alexander von Humboldt Foundation, as well as teaching excellence at UT. He has been a director and faculty member of the “Neural Systems and Behavior” summer course at the Marine Biological Laboratory in Woods Hole, Massachusetts (1995-2005), and the Chairman of international meetings including the “Gordon Research Conference in Neuroethology” at Oxford University (1999, 2002) and the International Society for Neuroethology (2010). He holds a position as Adjunct Scientist at the Marine Biological Laboratory in Woods Hole, MA. He was a visiting Fellow at the University of Cambridge (2012) and at the Ludwig Maximilian University and University of Konstanz in Germany (2015).
Research Summary:
Ion channels are fundamental for the workings of the nervous system. We study the function, regulation, and evolution of voltage-dependent ion channels. Our main focus has been to study the regulation of sodium and potassium channels by hormones such as testosterone, estrogen, and by phosphorylation. A major emphasis of the laboratory has been cloning these ion channel genes and understanding their transcriptional regulation. In addition, we have been studying the molecular evolution of ion channel genes in vertebrates.
-
2018 Thompson A, Infield DT, Smith AR, Smith GT, Ahern CA, Zakon HH Rapid evolution of a voltage-gated sodium channel gene in a lineage of electric fish leads to a persistent sodium current. PLoS Biology 16: e2004892.
2018 Immani S, Ghezzi A, Markham MR, Halling DB, Lu Y, Gallant J, Zakon HH Electrostatic Tuning of a Potassium Channel in Electric Fish. Current Biology 28: 2094-2102.
2017 Tarvin RD, Borghese C, Sachs W, Santos JC, Lu Y, O’Connell LA, Cannatella, DC, Harris RA, Zakon HH Interacting amino acid replacements allow poison frogs to evolve epibatidine resistance. Science 357:1261-1266.
2016 Tarvin RD, Santos JC, O’Connell LA, Zakon HH, Cannatella DC Convergent substitutions in a sodium channel suggest multiple origins of toxin resistance in poison frogs. Molecular Biology and Evolution 33: 1068-1081.
2015 Liebeskind BJ, Hillis DM, Zakon HH Convergence of ion channel gene content in early animal evolution. Proceedings of the National Academy of Sciences USA 112: E846-E851.
2014 Gallant JR, Traeger LL, Volkening JD, Moffett H, Chen PH, Novina CD, Phillips Jr. GN, Anand R, Wells GB, Pinch M, Robert Güth R, Unguez GA, Albert JS, Zakon HH, Samanta MP, Sussman MR Genomic basis for the convergent evolution of electric organs. Science 344: 1522-1525.
-

Our lab studies a number of questions using weakly electric fish as our model organism. These fish live in murky waters and are nocturnally active. They generate weak electric fields around themselves from a specialized electric organ and sense these electric fields, with specialized sensory receptors, called electroreceptors. They sense the distortions caused in their own electric fields to locate nearby objects, and the electric fields of other fish as communication signals.

Weakly Electric Fish
Weakly electric fish have evolved twice. One group, the mormyriformes, live in Africa, the other group, the gymnotiformes, live in South America.
Electric fish of both groups can be classified by their EODs into one of two types: "pulse" fish, which generate brief irregularly-occurring pulses, or "wave" fish, which generate highly regular sinusoidal EODs.
In the Zakon laboratory, we study South American wave fish. The EOD frequency of these wave fish is species specific, sexually dimorphic and individually distinct. The gold-lined knifefish (Sternopygus macrurus) generates EODs from 50-200 Hz, the glass knife (Eigenmannia virescens) has an EOD frequency range of 250-600 Hz; the brown ghost (Apteronotus leptorhynchus) fires at 650-1,100 Hz, and the black ghost (A. albifrons) from 800-1,200 Hz. Males discharge at a lower frequency than females in each of these species, except the brown ghost, where males are higher in frequency. We are able to alter the EOD frequency of individuals by treatment with sex steroids such as testosterone and estrogen.
Electric Organ
Electric organs (EOs) have evolved independently in at least six different groups of fish including two groups of elasmobranchs (Torpedo rays and the skates), and four groups of teleosts (gymnotiforms, mormyriformes, stargazers, catfish). In some groups EOs generate strong discharges (such as the Torpedo ray) and in others, weak discharges (such as the knifefish we study). In all but one case, they derive from muscle (the exception is the family Apteronotidae in which the axons of the EMNs form the electric organ). How the electric organ comes from muscle evolutionarily and developmentally is one question that we have been pursuing in our laboratory. The morphology of the electrocytes varies greatly between species and these variations are intimately bound up in the generation of species-specific electric organ discharge (EOD) waveforms. However, they operate on fundamental features of excitable membranes and current flow. Mainly, electric organs are composed of columns of electrocytes oriented in the same axis and ensheathed in high resistance connective tissue. The connective tissue channels the flow of current along the axis of the organ, out into the water, and back into the other end of the electric organ. Species such as Torpedo rays or electric eels, which have many stacks of flattened electrocytes, are capable of generating discharges of hundreds of volts. Weakly electric fish, such as the ones we study, make modest discharges of only hundreds of millivolts to a few volts.
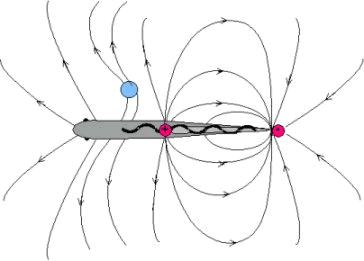 Schematic drawing of the electric field around a weakly electric fish. An object (blue area) with a higher conductivity than water increases the density of the iso-current density lines on the body surface of the fish, whereas a nonconducting object decreases their density (not shown). The undulating movement of the dorsal fin in gymnotids allows them to move without bending their tail and therefore minimizes the disturbance of the geometry of the electric field. This stabilizes the orientation of the electric organ (light gray area) and hence the orientation of the current density lines towards the electroreceptors, which is important for accurate electroreception (diagram drawn after Heiligenberg (1977)).
Schematic drawing of the electric field around a weakly electric fish. An object (blue area) with a higher conductivity than water increases the density of the iso-current density lines on the body surface of the fish, whereas a nonconducting object decreases their density (not shown). The undulating movement of the dorsal fin in gymnotids allows them to move without bending their tail and therefore minimizes the disturbance of the geometry of the electric field. This stabilizes the orientation of the electric organ (light gray area) and hence the orientation of the current density lines towards the electroreceptors, which is important for accurate electroreception (diagram drawn after Heiligenberg (1977)).
 Apteronotus leptorhynchus (Picture by J. Oestreich)
Apteronotus leptorhynchus (Picture by J. Oestreich)
-



