-

-

-
Seema Agarwala
Associate Professor
Molecular BiosciencesCellular and molecular bases of Neural Tube Closure and birth defectsagarwala@austin.utexas.edu
Phone: 512-232-4797
Office Location
PAT 407
Postal Address
2415 SPEEDWAY
AUSTIN, TX 78712-
Ph.D., State University of New York, Stony Brook (1990)
Research Summary:
My laboratory studies the cellular and molecular mechanisms involved in neural tube closure. During this process, a flat neural plate rolls up and fuses to form a closed neural tube. When this process goes awry, neural tube defects such as anencephaly and spina bifida can result. These defects are among the leading causes of congenital malformations and affect 1-5/1000 pregnancies.
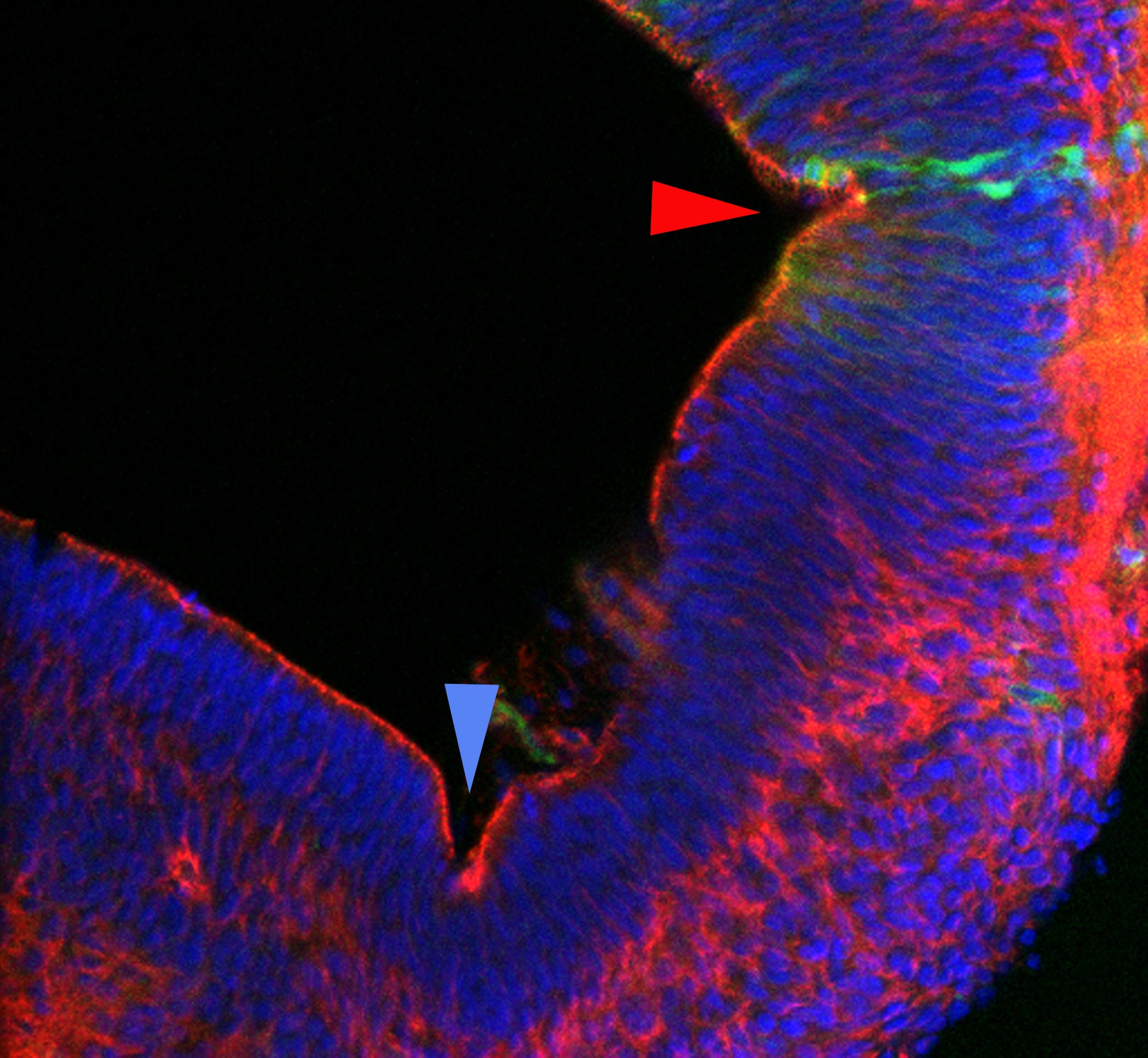 The tissue shape changes required for neural tube closure depend upon dynamic cell-shape changes and cell behaviors whose molecular bases are poorly understood. We have recently found that the molecular signals that regulate the specification of cell fates, also regulate many of the cell behaviors required for neural tube closure. For example, Bone Morphogenetic Proteins (BMPs) can induce columnar cells at the ventral midline to constrict asymmetrically and become wedge-shaped. This event buckles the ventral midline of the neural plate and elevates the neural folds so that they can ultimately fuse across the dorsal midline. Though conceptually simple, this process involves BMP interactions with the cell's polarity pathways, cellular cytoskeleton and the cell cycle machinery. We approach this problem in amniotes (mouse, chick) by using both in vivo and in vitro manipulations, high-resolution microscopy, real-time imaging and biochemical analyses. Using similar techniques, we have also begun to look at how two discrete neural folds fuse with one another to form a closed and continuous neural tube. Finally, we are also examining the role of surrounding tissues (epidermal ectoderm, mesenchyme, notochord) in closing the neural tube.
The tissue shape changes required for neural tube closure depend upon dynamic cell-shape changes and cell behaviors whose molecular bases are poorly understood. We have recently found that the molecular signals that regulate the specification of cell fates, also regulate many of the cell behaviors required for neural tube closure. For example, Bone Morphogenetic Proteins (BMPs) can induce columnar cells at the ventral midline to constrict asymmetrically and become wedge-shaped. This event buckles the ventral midline of the neural plate and elevates the neural folds so that they can ultimately fuse across the dorsal midline. Though conceptually simple, this process involves BMP interactions with the cell's polarity pathways, cellular cytoskeleton and the cell cycle machinery. We approach this problem in amniotes (mouse, chick) by using both in vivo and in vitro manipulations, high-resolution microscopy, real-time imaging and biochemical analyses. Using similar techniques, we have also begun to look at how two discrete neural folds fuse with one another to form a closed and continuous neural tube. Finally, we are also examining the role of surrounding tissues (epidermal ectoderm, mesenchyme, notochord) in closing the neural tube.
-
Research Summary:
My laboratory studies the cellular and molecular mechanisms involved in neural tube closure. During this process, a flat neural plate rolls up and fuses to form a closed neural tube. When this process goes awry, neural tube defects such as anencephaly and spina bifida can result. These defects are among the leading causes of congenital malformations and affect 1-5/1000 pregnancies.
The tissue shape changes required for neural tube closure depend upon dynamic cell-shape changes and cell behaviors whose molecular bases are poorly understood. We have recently found that the molecular signals that regulate the specification of cell fates, also regulate many of the cell behaviors required for neural tube closure. For example, Bone Morphogenetic Proteins (BMPs) can induce columnar cells at the ventral midline to constrict asymmetrically and become wedge-shaped. This event buckles the ventral midline of the neural plate and elevates the neural folds so that they can ultimately fuse across the dorsal midline. Though conceptually simple, this process involves BMP interactions with the cell's polarity pathways, cellular cytoskeleton and the cell cycle machinery. We approach this problem in amniotes (mouse, chick) by using both in vivo and in vitro manipulations, high-resolution microscopy, real-time imaging and biochemical analyses. Using similar techniques, we have also begun to look at how two discrete neural folds fuse with one another to form a closed and continuous neural tube. Finally, we are also examining the role of surrounding tissues (epidermal ectoderm, mesenchyme, notochord) in closing the neural tube.
-
Selected Publications:
1. Eom, D.S., Amarnath, S., Fogel, J.L., and Agarwala, S. (2013). Apicobasal polarity and neural tube closure. Dev. Growth. Differ. 55:164-72.
2. Eom, D.S., Amarnath, S., Fogel, J.L., and Agarwala, S.(2012).Bone morphogenetic proteins regulate hinge point formation during neural tube closure by dynamic modulation of apicobasal polarity. Brain Defects Research (part A). epub ahead of print: doi: 10.1002/bdra.23052. Invited Article;
Awarded Best Paper of the Year (2012) Award by the Teratolgoy Society, 2013.
3. Brown, CB, Eom, D.S., Amarnath, S., and Agarwala, S. (2012). In vivo electroporation of E1 chick embryos. CSH Protocols epub ahead of print, doi:10.1101/pdb.prot069708.
4. Eom, D.S., Amarnath, S., Fogel, J.L., and Agarwala, S. (2011). Bone Morphogenetic proteins (BMPs) regulate neural tube closure by interacting with the apicobasal polarity pathway. Development,138: 3179-3188. Featured article in Development.
Selected by Faculty of 1000 Biology: Watanabe Y, Nakamura H: 2011. F1000.com/11942956. http://f1000.com/11942956
5. Hasan, K.B., Agarwala, S., and Ragsdale, C, W (2010). PHOX2A is a primary regulator of oculomotor complex nucleogenesis. Development. 137:1205-13.
Selected by Faculty of 1000 Biology: Brunet J: 2010. F1000.com/2663957; and Kiecker C, Lumsden A: 2010. F1000.com/2663957. http://f1000biology.com/article/id/2663957/evaluation
6. Agarwala, S and Ragsdale, C.W., (2008). Neural Patterning:Rostral-Caudal Patterning:Midbrain Patterning. In L. Squires (Ed. In chief), Enclopedia of Neuroscience, Academic Press, Oxford, 2008.
7. Fogel., J.L., Chiang, C., and Agarwala, S. (2008). Dynamic Size Regulation and Residual Ventral Cell-Fate Specification in the mouse midbrain in the absence of Sonic Hedgehog. Dev. Dynamics. 237:1359-1372.
8. Bayly R.D., Ngo, M, Aglyamova, G.V., Fogel, J.L., Agarwala, S (2007). Regulation of Ventral Midbrain Patterning by Hedgehog Signaling, Development 134: 2115-2124
9. Agarwala, S. and Ragsdale, C.W (2002). A role for midbrain arcs in nucleogenesis., Development 129: 5779-5788
10. Agarwala, S., Sanders, T.A., and Ragsdale, C.W (2001). Sonic Hedgehog control of size, shape and position in midbrain pattern formation., Science 291: 2147-2149.
-
1. 2013 James G. Wilson Publication Award ($1,500). The Teratology Society (given to the best publication in Birth Defects Research), 53rd Annual Meeting of the Teratology Society, Tucson, Arizona.
2. 2011 Eom, D.S., Amarnath, S., Fogel, J.L., and Agarwala, S. Bone Morphogenetic proteins (BMPs) regulate neural tube closure by interacting with the apicobasal polarity pathway. Development,138: 3179-3188.
Featured article in Development.
Selected by Faculty of 1000 Biology: Watanabe Y, Nakamura H: 2011. F1000.com/11942956. http://f1000.com/11942956
2010 Hasan, K.B., Agarwala, S., and Ragsdale, C, W (2010). PHOX2A is a primary regulator of oculomotor complex nucleogenesis. Development. 137:1205-13.
Selected by Faculty of 1000 Biology: Brunet J: 2010. F1000.com/2663957; and Kiecker C, Lumsden A: 2010. F1000.com/2663957. http://f1000biology.com/article/id/2663957/evaluation
2007 Teaching Excellence Award; College of Natural Sciences; University of Texas, Austin.
2006 The Rom Rhone Fellowship for travel to International Meetings ($ 1,000).
-
2013 Invited Talk:Cell cycle dependent BMP-TGFß-apicobasal polarity protein interactions in regulating neural tube closure. 8th International Conference on Neural Tube Defects, AT&T Center, Austin,Texas.
2013 Invited Talk: Apicobasal polarity and neural tube closure Minisymposium:. Morphogenesis and fate specification of the ventral midbrain floor plate. Chair: Raj Awatramani; Annual Meeting, Society for Neuroscience, San Diego.
2013 Invited Talk: “Bone Morphogenetic Proteins Regulate Hinge Point Formation during Neural Tube Closure by Dynamic Modulation of Apicobasal Polarity” In: The plenary session for the James G. Wilson Publication Award, 53rd Annual Meeting of The Teratology Society, Tucson, Arizona.
2012 Invited Talk: Antagonistic BMP-TGFß interactions with the apicobasal polarity pathway regulate midbrain neural tube closure. In: “Using chicks for Cell Biology, (C. Kalcheim and G. Sheng, chairs) 7th International Chick Meeting, Nagoya, Japan.
2012 Bone morphogenetic protein modulation directs neural tube closure by dynamically regulating apicobasal polarity in "Neurulation and Neural Tube Defects" (A. Copp, Chair). International Society of Developmental Neuroscience's (ISDN), Tata Institute of Fundamental Research in Mumbai, India.
2011 Invited Talk: FOXA2 directs TGFß-BMP interactions with the apicobasal polarity pathway during neural tube closure; 7th International Conference on Neural Tube Defects, AT&T Center, Austin, Texas.
2010 Bone Morphogenetic Proteins direct neural tube closure by regulating apicobasal polarity. SDB Regional Southwestern Meeting, Austin Texas.
2008 BMP signaling in patterning and closing the vertebrate midbrain. Katholiecke Universiteit, Leuven, Belgium.
-
Graduate Cou 1. Graduate Course: Bio 381K.7 Cellular and Molecular Bases of Neural Development (Previously,Development and Plasticity of Nervous System).
2. Undergraduate Course: 365N Cellular and Molecular bases of Neural Development (Previously Development and Plasticity of the Nervous System)
This course has a writing flag.
-



